The formulary resources listed below have been provided to assist healthcare decision makers with information needed to learn more about AURLUMYN.
AURLUMYN is the first and only FDA-approved treatment option for severe frostbite in adults to reduce the risk of digit amputation
Effectiveness was established in young, healthy adults who suffered frostbite at high altitudes.
Key Product Information

Prescribing Information
The most current full Prescribing Information for AURLUMYN.

Commercial Availability Press Release
Press release sharing the commercial availability of AURLUMYN for use in the U.S.
Formulary Toolkit Resources

Formulary Presentation
Outlines the value and supporting clinical data behind AURLUMYN as a treatment for severe frostbite.

Product Monograph
Provides key information about AURLUMYN’s efficacy and safety data, clinical pharmacology, dosing and administration, and other important product details to help guide formulary decisions and inform end users.

AMCP Dossier (Available by request only)
This document provides information on the clinical and economic aspects of AURLUMYN, leveraging clinical data and the FDA-approved label.
Published Literature

Bibliography
Provides literature relevant to AURLUMYN and its supporting clinical data (eg, peer-reviewed articles, guideline recommendations) and includes frostbite-related literature for context.
Distribution, Pricing and Reimbursement Information

Ordering, Pricing and Distribution Fact Sheet
Lists practical AURLUMYN ordering, pricing, and distribution details. Includes NDC, WAC pricing, storage, and handling information.
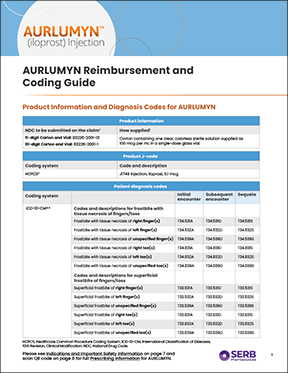
Reimbursement and Coding Guide
Provides information to help with AURLUMYN reimbursement, including key product information and potential diagnosis codes. Provides background on how new technology add-on payments (NTAP) and outlier payments may offset treatment expenses.
FDA, US Food and Drug Administration; NDC, National Drug Code; rt-PA, recombinant tissue plasminogen activator; WAC, wholesale acquisition cost.
